Progesterone levels: normal levels, during pregnancy, low levels

Reviewed by Steve Silvestro, MD, Ro,
Written by Nancy LaChance, BSN, RN
last updated: Jan 26, 2022
5 min read
Here's what we'll cover
Hormones play an important role in how our bodies function, including reproductive health. Progesterone is one of the key hormones that support female fertility, menstruation, and carrying a pregnancy to term. It even plays a role in male reproduction. Here, we’ll discuss more details about what progesterone is, how its levels change, what it means for health and fertility if progesterone levels are low, and what you can do about it.
What is progesterone?
Progesterone is a sex hormone that plays a key role in menstruation, fertility, and pregnancy. It is created in the brain's pituitary gland and in the ovaries after ovulation. Progesterone levels rise and fall throughout a woman’s monthly cycle and pregnancy. It even plays an important role in male fertility by assisting in sperm development (Cable, 2021).
What does progesterone do?
Progesterone is sometimes called “the pregnancy hormone” because of the vital role it plays in the body’s ability to become and stay pregnant (Di Renzo, 2016). It plays an essential part in the menstrual cycle and preparing the body to carry a fertilized egg.
Menstruation, fertility, and pregnancy
To understand the role of progesterone in fertility and pregnancy, it helps to start by reviewing its role in the menstrual cycle.
The ovary will send an egg out to be fertilized in the uterus during the menstrual cycle. The spot on the ovary that ruptured to release the egg becomes a small, temporary cyst called a corpus luteum. The main job of the corpus luteum is to produce progesterone to thicken the uterine lining and prepare it to receive a fertilized egg (Cable, 2021).
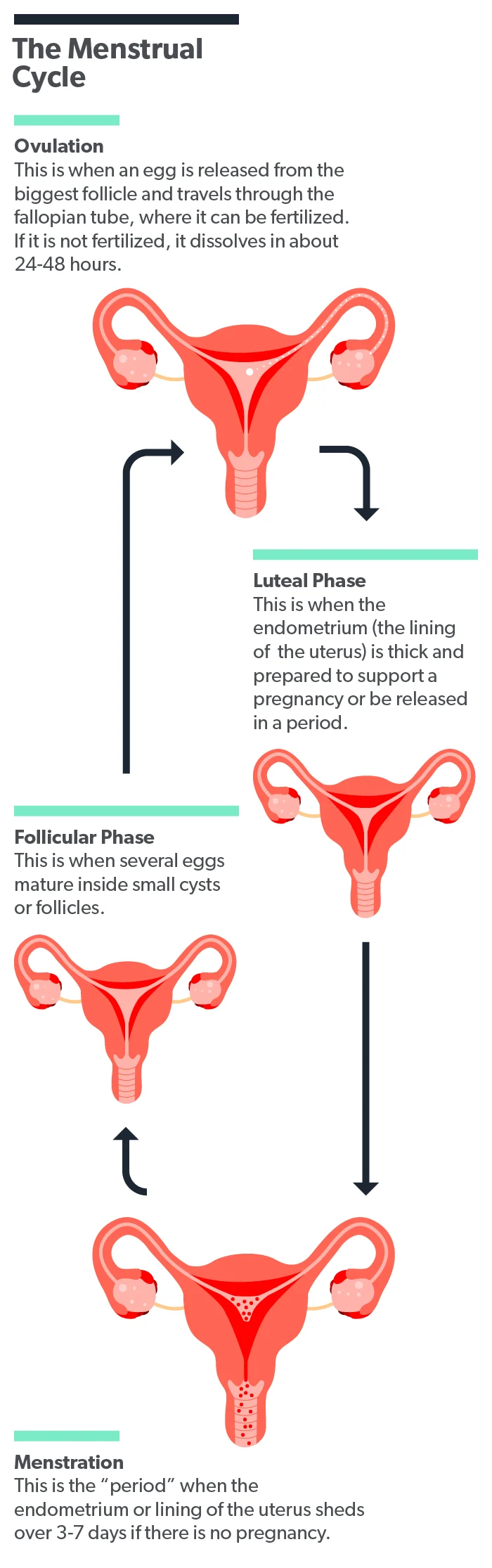
Once a person becomes pregnant, progesterone released by the corpus luteum continues to play a key role. Progesterone is essential for a pregnancy to survive, and the corpus luteum continues to produce it for the first few months of pregnancy until the placenta is established and can take over progesterone production (Oliver, 2021).
Contraception and medical therapies
Progesterone also plays a big role in medications designed to prevent pregnancy or relieve symptoms of menopause, irregular periods, endometriosis, and other reproductive issues. The form of progesterone in these pharmaceuticals is called progestin—it’s the synthetic form of progesterone and has the same effect on the human body.
Progestin is an ingredient in most hormonal contraceptives, including combined birth control pills, progestin-only pills, hormonal IUDs, contraceptive rings and patches, and the depo shot. When the body receives a constant dose of progestin (rather than the peaks and valleys that occur in a normal cycle), ovulation doesn’t occur, and the lining of the uterus remains thin, rather than thickened and ready for a fertilized egg to implant (Edwards, 2021; Oliver, 2021).
Taking progestin can also relieve symptoms of menopause, help regulate irregular periods, and reduce pain associated with endometriosis. Most women can take progestin therapeutically, but some health conditions make it unsafe. If you have questions about it, your healthcare provider is the best person to help you decide whether a progestin-containing medication is right for you (Edwards, 2021).
Progesterone levels and pregnancy
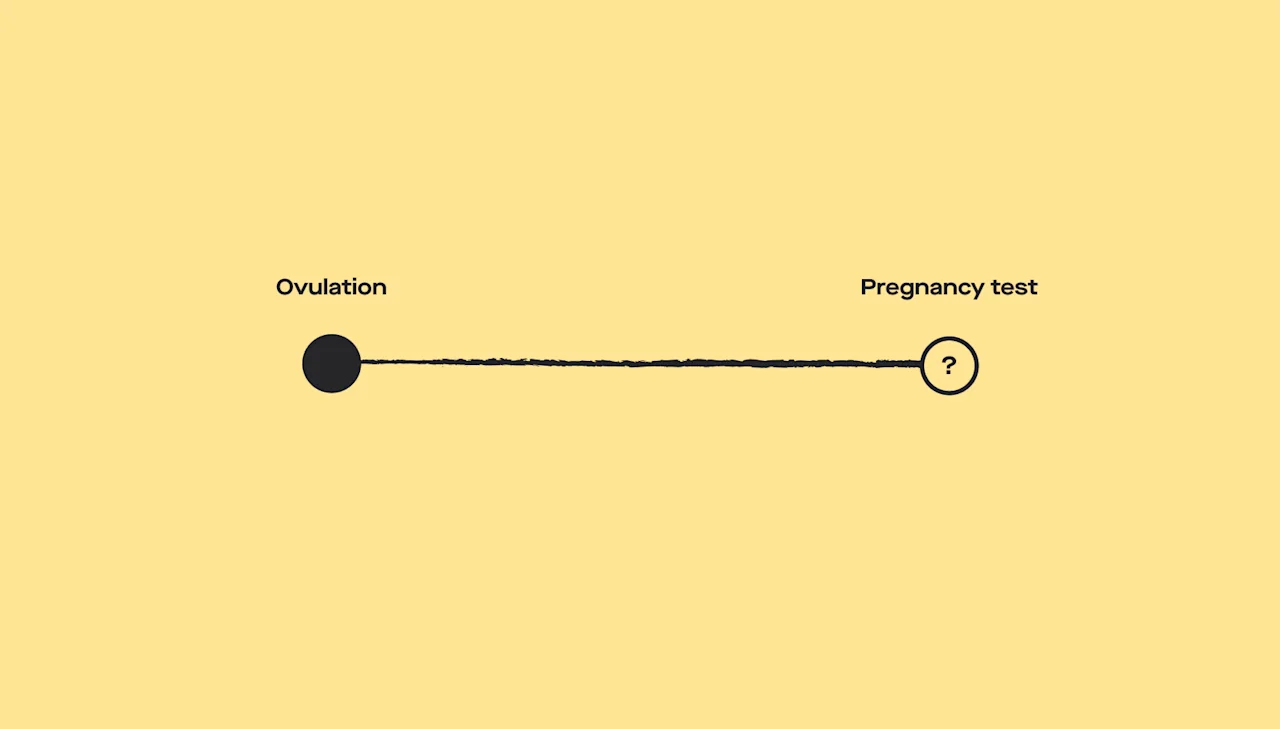
The role of progesterone is important for pregnancy from conception to delivery. For conception to occur, progesterone levels must be high enough during the menstrual cycle for the uterus to accept and maintain a fertilized egg (Oliver, 2021). If progesterone levels are too low, the uterus can’t do this.
During pregnancy, progesterone promotes good blood flow to the fetus, helps the uterus stay relaxed as it grows, prevents lactation from occurring, and helps the immune system protect the pregnancy (Cable, 2021).
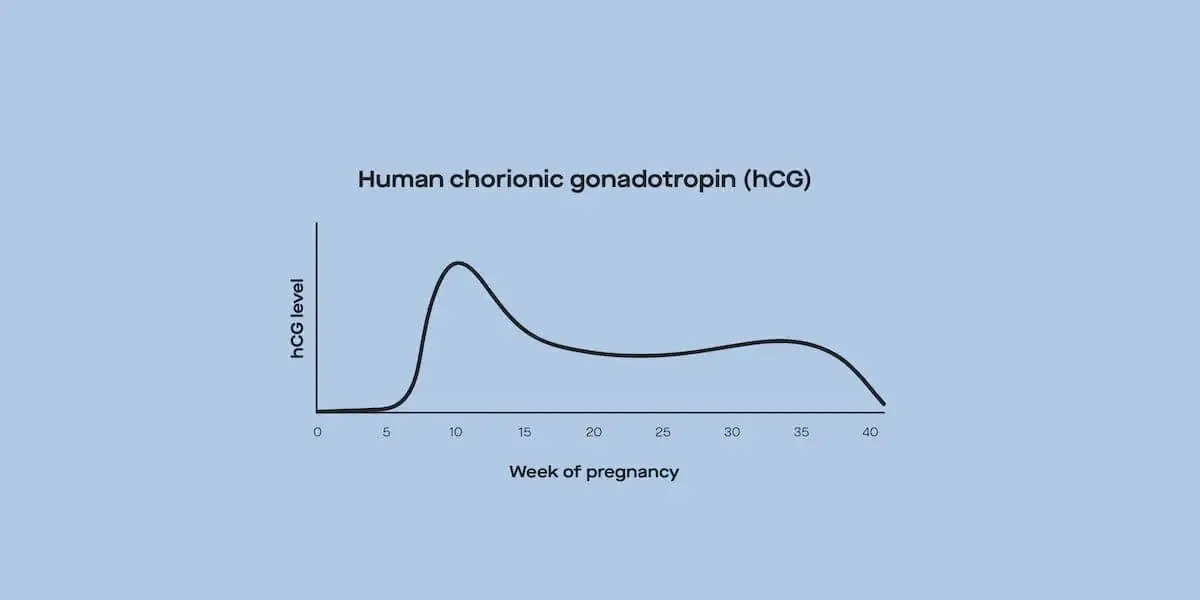
Progesterone levels rise steadily before evening out and ultimately dropping in the last few weeks of pregnancy. Since the corpus luteum produces progesterone for the first trimester of pregnancy, a problem with the corpus luteum can cause early pregnancy problems (like spotting, pain, or miscarriage); this is estimated to cause 35% of recurrent miscarriages (Taraborrelli, 2015).
Late in pregnancy and after childbirth, progesterone levels drop sharply, allowing the body to produce milk for the baby (Cable, 2021).
What are normal progesterone levels?
Why would you check your progesterone level? Unless you are experiencing symptoms such as moodiness, fatigue, abnormal periods, infertility, or difficulties staying pregnant, you probably don’t need to. If you do, your healthcare provider may want to check your hormone levels, including progesterone, estrogen, and human chorionic gonadotropin (hCG). Let’s look at normal progesterone levels and explore when getting your levels checked may be a good idea.
Here are the normal serum progesterone levels in adult females (meaning the concentration of progesterone detected in a blood sample) (Stricker, 2006):
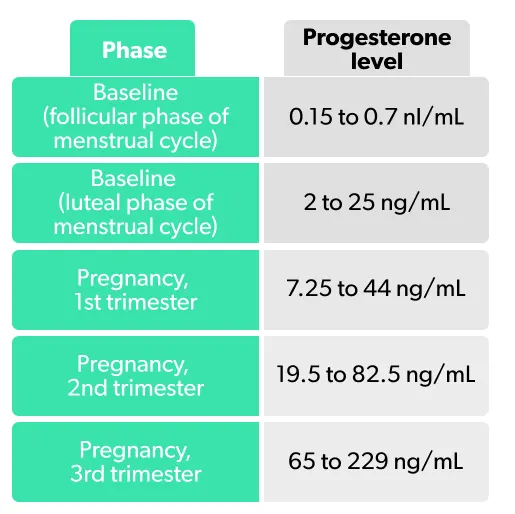
High progesterone levels tend not to be a medical issue and are usually a sign that ovulation has occurred and that a person is pregnant (Cable, 2021).
Symptoms of low progesterone
Women who have low levels of progesterone may experience (Cable, 2021):
Abnormal menstrual symptoms (like spotting, heavy periods, and irregular periods)
Difficulty getting pregnant
Spotting or abdominal pain in early pregnancy
Repeated early pregnancy loss
What can cause low progesterone?
Low progesterone may signify an underlying health issue or environmental factors. Fortunately, many of these conditions are relatively common and have a range of treatment options. Some of these conditions include:
Chronic stress
Some stress in daily life is normal, but extended periods of intense stress and anxiety cause the body to produce high amounts of corticosteroids (stress hormones). This spike in stress hormones can cause a cascade of other bodily functions to shift and go into “fight or flight” mode. Excessive corticosteroid production represses progesterone production and makes the uterus less receptive to it (Turankar, 2013).
Ectopic pregnancy
Ectopic pregnancy, when a fertilized egg implants in a location other than the uterus (like the cervix or fallopian tubes), can be a reason for low progesterone levels (Cable, 2021). Ectopic pregnancies are dangerous and need to be promptly addressed by a healthcare provider. Most women experience clear symptoms, such as pain, vaginal bleeding, and nausea, and can receive early and effective treatment (Mummert, 2021).
Hypothyroidism
Hypothyroidism is a condition in which the thyroid gland (located in the neck) doesn’t produce enough of the key hormones it is responsible for creating. This can lead to a hormonal imbalance that can include low progesterone and result in women ovulating irregularly (Turankar, 2013).
Hyperprolactinemia
Hyperprolactinemia is a common hormonal disorder related to hypothyroidism; it occurs when the thyroid gland and the pituitary gland are not working correctly together, resulting in high blood levels of the hormone prolactin, and lowered levels of progesterone (Samperi, 2019).
PCOS
Polycystic ovary syndrome (PCOS) is a common hormonal disorder affecting young women. It can cause multiple small cysts on the ovaries, over-production of androgens (male sex hormones), and under-production of hormones like progesterone. This can cause period irregularities, a risk of miscarriage, and fertility issues (Maruthini, 2014; Rasquin, 2021).
Treatment for low progesterone
Having low progesterone levels can be physically uncomfortable and emotionally stressful, particularly if a person is trying or planning to have a baby. It’s important to remember that low progesterone and conditions that cause it are reasonably common. Many women with these conditions can become pregnant and have healthy pregnancies and babies. There are a variety of resources and treatments to help deal with it, including progesterone supplements (usually in the form of creams, suppositories, injections, or pills). Side effects tend to be mild and similar to that of progestin-based birth control methods (Edwards, 2021; Tanbo, 2018).
If you think you may be experiencing symptoms of low progesterone, the first step is to reach out to your healthcare provider. They can help answer questions specific to your situation, find out if there is an underlying medical condition, and take the next steps towards testing and treatment.
DISCLAIMER
If you have any medical questions or concerns, please talk to your healthcare provider. The articles on Health Guide are underpinned by peer-reviewed research and information drawn from medical societies and governmental agencies. However, they are not a substitute for professional medical advice, diagnosis, or treatment.
Cable, J. K. & Grider, M. H. (2021). Physiology, progesterone. [Updated May 9, 2021]. In: StatPearls [Internet]. Retrieved on Jan. 18, 2022 from https://www.ncbi.nlm.nih.gov/books/NBK558960/
Di Renzo, G. C., Giardina, I., Clerici, G., Brillo, E., & Gerli, S. (2016). Progesterone in normal and pathological pregnancy. Hormone Molecular Biology Clinical Investigation, 1 ;27(1):35-48. doi: 10.1515/hmbci-2016-0038. Retrieved from https://pubmed.ncbi.nlm.nih.gov/27662646/
Edwards, M. & Can, A. S. (2021). Progestin. [Updated Sep 22, 2021]. In: StatPearls [Internet]. Retrieved on Jan. 18, 2022 from https://www.ncbi.nlm.nih.gov/boo k s/NBK563211/
Maruthini, D., Harris, S. E., Barth, J. H., Balen, A. H., Campbell, B. K., & Picton, H. M. (2014). The effect of metformin treatment in vivo on acute and long-term energy metabolism and progesterone production in vitro by granulosa cells from women with polycystic ovary syndrome. Human Reproduction (Oxford, England) , 29 (10), 2302–2316. doi: 10.1093/humrep/deu187. Retrieved from https://pubmed.ncbi.nlm.nih.gov/26187711/
Mummert, T. & Gnugnoli, D. M. (2021). Ectopic pregnancy. [Updated Dec 9, 2021]. In: StatPearls [Internet]. Retrieved on Jan. 18, 2022 from https://www.ncbi.nlm.nih.gov/books/NBK539860/
Oliver, R. & Pillarisetty, L. S. (2020). Anatomy, abdomen and pelvis, ovary corpus luteum. [Updated Nov 3, 2020]. In: StatPearls [Internet]. Retrieved on Jan. 18, 2022 from https://www.ncbi.nlm.nih.gov/books/NBK539704/
Rasquin, L., Anastasopoulou, C., & Mayrin, J. V. (2021). Polycystic ovarian disease. [Updated Jul 21, 2021]. In: StatPearls [Internet]. Retrieved on Jan. 18, 2022 from https://www.ncbi.nlm.nih.gov/books/NBK459251/
Samperi, I., Lithgow, K., & Karavitaki, N. (2019). Hyperprolactinaemia. Journal of Clinical Medicine , 8 (12), 2203. doi: 10.3390/jcm8122203 . Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6947286/
Stricker, R., Eberhart, R., Chevailler, M.C., Quinn, F.A., Bischof, P., & Stricker, R. (2006). Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clinical Chemistry and Laboratory Medicine, 44 (7):883-7. doi: 10.1515/CCLM.2006.160. Retrieved from https://pubmed.ncbi.nlm.nih.gov/16776638/
Tanbo, T., Mellembakken, J., Bjercke, S., Ring, E., Åbyholm, T., Fedorcsak, P. (2018). Ovulation induction in polycystic ovary syndrome. Acta Obstetricia et Gynecologica Scandinavica, 97 (10):1162-1167. doi: 10.1111/aogs.13395. Retrieved from https://obgyn.onlinelibrary.wiley.com/doi/10.1111/aogs.13395
Taraborrelli, S. (2015). Physiology, production and action of progesterone. Acta Obstetetricia et Gynecologica Scandinavica, 94, 161:8-16. doi: 10.1111/aogs.12771. Retrieved from https://pubmed.ncbi.nlm.nih.gov/26358238/
Turankar, S., Sonone, K., & Turankar, A. (2013). Hyperprolactinaemia and its comparison with hypothyroidism in primary infertile women. Journal of Clinical and Diagnostic Research: JCDR , 7 (5), 794–796. doi: 10.7860/JCDR/2013/4878.2941. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3681039/