Fertility testing: what to expect

Reviewed by Chimene Richa, MD,
Written by Frances Gatta
last updated: Jun 07, 2022
5 min read
Here's what we'll cover
Fertility testing can reveal what’s preventing you from conceiving in most cases. If you’ve had unprotected sex for 12 months and have not achieved pregnancy, speak with a healthcare provider about fertility testing.
Because fertility declines with age, experts recommend that people 35 years and older wait for six months before getting tested (ASRM, 2022). Here’s what you need to know about fertility testing for men and women.
Diagnosing infertility
The first step is visiting a healthcare provider who can assess your medical history and look for risk factors or any underlying health issues that could affect your fertility. Things a provider might ask you include:
General information: This includes age, how long you’ve been trying to have a baby, if you’ve had fertility issues with previous partners, and more.
Medical history: This is anything related to your physical health. It could include health conditions you had as a child, medications, past surgeries, and reproductive or urinary tract infections. They also might ask if you use any contraceptives or have had a sexually transmitted infection (STI).
Family history: Genetics can play a role in fertility. You’ll likely be asked if anyone in your family has experienced fertility issues, pregnancy loss, genetic disorders, and more.
Lifestyle habits: This goes over potential risk factors like smoking, poor diet, stress levels, exposure to toxins, and more.
Sexual history: This part is to understand when and how often you have sex. Your healthcare may ask about your libido (sex drive), sexual performance, and any problems with erections or ejaculation (Ferlin, 2020).
Following an evaluation, a thorough physical exam is done to screen for STIs, androgen deficiencies (lower male sex hormones), and anything else that may be affecting your fertility.
Fertility testing for men
Research shows that male factors contribute to infertility in 50% of couples trying to get pregnant. Male infertility affects up to 7% of men globally (Boitrelle, 2021; Agarwal, 2021; Houston, 2021).
Here’s a look at what’s involved with fertility testing for men:
Semen analysis
This lab test is the first and most basic step in evaluating male fertility problems. A semen analysis provides insight into the health of your testes, seminal tract, and checks for the following (Boitrelle, 2021; Ferlin, 2020):
Semen volume
Total sperm number
Sperm concentration
Sperm vitality (percentage of live sperm)
Sperm movement (motility)
Sperm shape (morphology)
The test itself requires a semen sample for lab testing. You can either ejaculate into a container in a private room at a doctor's office or use a special condom during sex.
Further male fertility testing
After initial evaluations, you may need to go through the following for your provider to better understand your fertility status (Ferlin, 2020):
Post-ejaculation urinalysis: As the name implies, this test is carried out after ejaculation. It shows whether a person has retrograde ejaculation, which is when sperm swim backward into the bladder instead of out through the penis.
Imaging: A doctor may conduct an ultrasound to check for abnormalities with your scrotum or rectum that could contribute to infertility.
Hormone tests: These check hormones that play a role in sperm production including follicle-stimulating hormone (FSH), luteinizing hormone (LH), prolactin, and testosterone levels.
Antisperm antibody testing: This test screens for antibodies that could be fighting against sperm preventing fertility. Antisperm antibodies—which develop when sperm comes in contact with the immune system—are present in 9–12% of those with infertility (Vickram, 2019).
Genetic testing: This checks for genetic factors that may cause infertility. Genetic testing is especially recommended in cases where previous tests reveal severe oligozoospermia (low sperm count) or azoospermia (no sperm) (Kuroda, 2020).
Testicular biopsy: This checks to see if sperm production is normal in the testes. If so, there may be a problem moving sperm out of testes, like a blockage.
Fertility testing for women
For women struggling to conceive, the first steps are meeting with a healthcare provider who will assess your medical history and perform a basic gynecological exam.
Some details you may be asked include your age, how long you’ve been trying to have a baby, your menstrual cycle, and more. After this, the next step is ovulation testing (Walker, 2021).
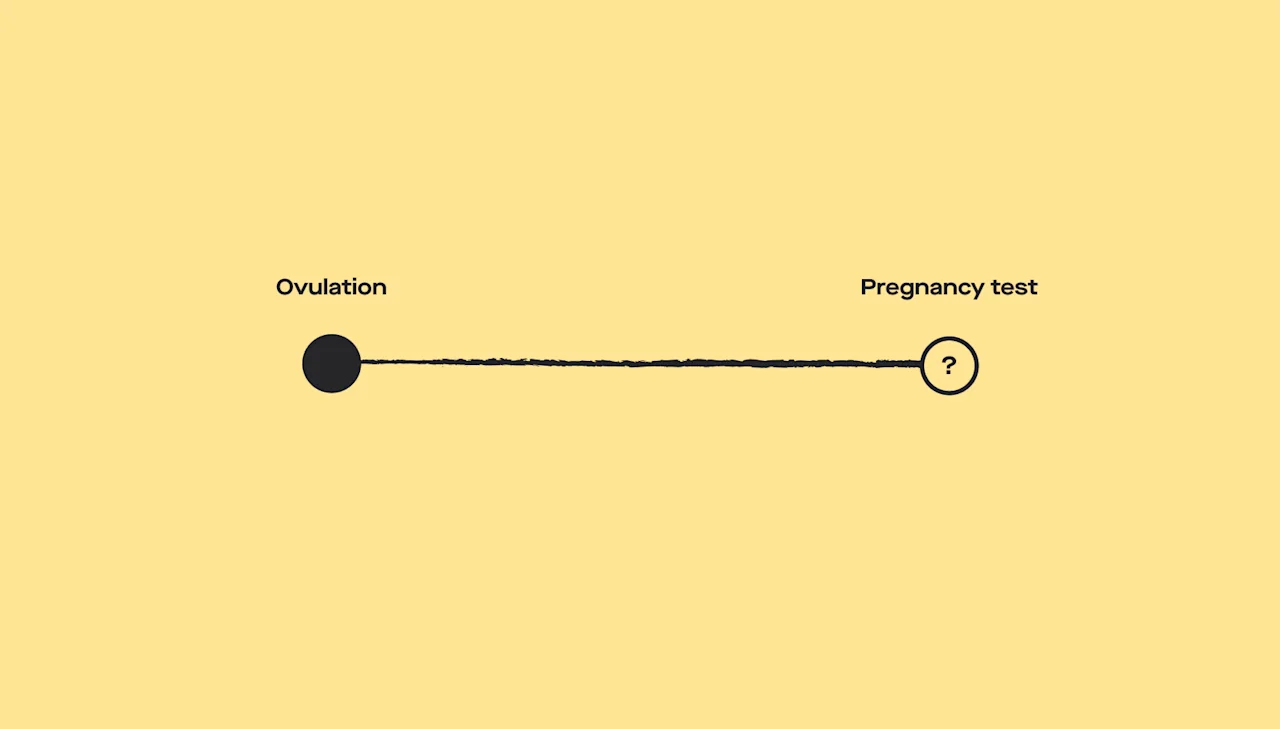
Ovulation testing
Getting pregnant depends on ovulation, which is the point in a woman’s menstrual cycle where an egg is released for fertilization. Ovulation problems are the most common cause of infertility in women.
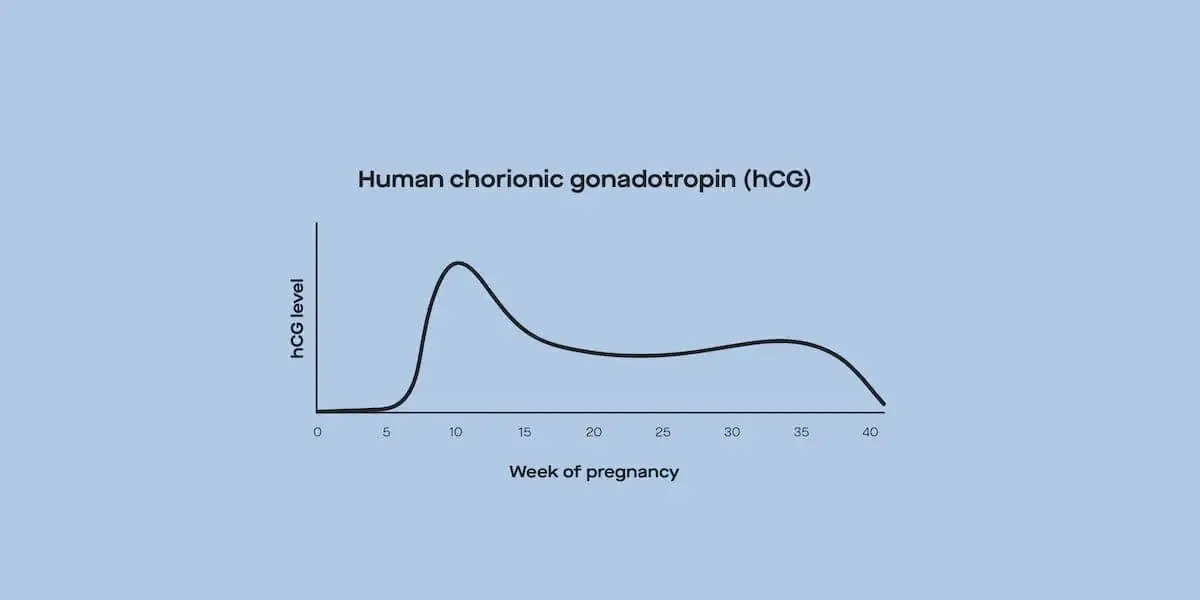
A blood or urine test can tell you more about how your ovaries are functioning. These tests check the levels of hormones responsible for ovulation, like progesterone and LH (Walker, 2021).
Ovarian reserve testing
If your ovulation tests come back normal, the next step is ovarian reserve testing. This checks the quantity and quality of eggs in your ovaries. These tests also assess hormones including FSH, estradiol, and anti-müllerian hormone (AMH) (Preston, 2019).
Other female fertility tests
Beyond the ovaries, other tests may be recommended to check for abnormalities in the uterus, pelvis, and other reproductive organs.
Some other useful tests for infertility diagnosis include (Walker, 2021):
Ultrasound: This type of imaging is helpful for checking the ovaries and uterus for abnormalities or conditions that may reduce fertility including endometriosis, fibroids, and polycystic ovary syndrome (PCOS) (Kondogari, 2022).
Hysterosalpingogram (HSG)This type of X-ray looks for blockages, tumors, and other problems that impact the uterus or fallopian tubes.
Hysteroscopy: Experts consider this procedure the gold standard for checking the uterine walls for abnormalities. A doctor inserts a hysteroscope (a thin telescope-like device with a light and camera on it) through the vagina to look inside the uterus.
Laparoscopy: This is a minimally invasive surgery to help your provider detect conditions contributing to infertility like endometriosis or pelvic adhesions. The procedure involves making a small cut in the lower belly so that a laparoscope (a small camera) can be used to view the pelvic organs.
Where can I get a fertility test done?
Depending on what kind of test you need, you can get examined at a clinic or take an at-home fertility test. These kits are helpful for things like hormone testing or semen analysis. Some pros of at-home tests are they’re simple, discreet, time-saving, and cost-effective (Gonzalez, 2021).
All you have to do is purchase a test kit, take a sample, and then mail it to a lab according to the instructions. Results are usually available in a few days. If you’re having trouble interpreting the results or think you need further fertility testing, reach out to a healthcare provider who can recommend next steps.
When to see someone about fertility testing
If you’re younger than 35 and have been trying unsuccessfully to have a baby for at least 12 months, you might want to see a medical professional about fertility testing. Those 35 years and older can get tested after six months of having regular unprotected sex that doesn’t result in pregnancy (ASRM, 2022).
Both male and female reproductive systems have to be in good condition to have a baby. If you’re in a couple, it’s best to involve them when considering fertility testing. Assessing the health of all parties involved in pregnancy is the best way to figure out what fertility issues exist––and the next steps for treating them.
DISCLAIMER
If you have any medical questions or concerns, please talk to your healthcare provider. The articles on Health Guide are underpinned by peer-reviewed research and information drawn from medical societies and governmental agencies. However, they are not a substitute for professional medical advice, diagnosis, or treatment.
Agarwal, A., Bakaran, S. Parekh, N., et al. (2021). Male infertility. The Lancet, 397, 23-29. Retrieved from https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32667-2/fulltext
Ammar, T., Sidhu, P. S., & Wilkins, C. J. (2012). Male infertility: the role of imaging in diagnosis and management. The British Journal of Radiology , 85 (1), S59–S68. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3746399/
Boitrelle, F., Shah, R., Saleh, R., et al. (2021). The Sixth Edition of the WHO Manual for Human Semen Analysis: A Critical Review and SWOT Analysis. Life (Basel, Switzerland) , 11 (12), 1368. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8706130/
Esteves, S. C., Miyaoka, R., & Agarwal, A. (2011). An update on the clinical assessment of the infertile male. [corrected]. Clinics , 66 (4), 691–700. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3093801/
Ferlin, A. & Foresta, C. (2020). Infertility: Practical Clinical Issues for Routine Investigation of the Male Partner. Journal of Clinical Medicine , 9 (6), 1644. Retrieved from https://www.mdpi.com/2077-0383/9/6/1644
Gelbaya, T. A., Potdar, N., Jeve, Y. B., et al. (2014). Definition and epidemiology of unexplained infertility. Obstetrical & Gynecological Survey , 69 (2), 109–115. Retrieved from https://pubmed.ncbi.nlm.nih.gov/25112489/
Gonzalez, D., Narasimman, M., Best, J. C., et al. (2021). Clinical Update on Home Testing for Male Fertility. The World Journal of Men's Health , 39 (4), 615–625. Retrieved from https://pubmed.ncbi.nlm.nih.gov/33474845/
Houston, B., Riera-Escamilla, A., Wyrwoll M., et al. (2021). A systematic review of the validated monogenic causes of human male infertility: 2020 update and a discussion of emerging gene-disease relationships. Human Reproduction Update , 28 (1), 15–29, Retrieved from https://academic.oup.com/humupd/article/28/1/15/6366465?login=true
Kondagari, L., Kahn, J., & Singh, M. (2022). Sonography Gynecology Infertility Assessment, Protocols, And Interpretation. StatPearls. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK572093/
Kuroda, S., Usui, K., Sanjo, H., et al. (2020). Genetic disorders and male infertility. Reproductive Medicine and Biology , 19 (4), 314–322. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7542010/
Parry, J. P. & Koch, C. A. (2019). Ovarian Reserve Testing. StatPearls . Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK279058/
Practice Committee of the American Society for Reproductive Medicine (ASRM) and the Society for Reproductive Endocrinology and Infertility. (2022). Optimizing natural fertility: a committee opinion. Fertility and Sterility, 117 (1), 53–63. Retrieved from https://www.asrm.org/globalassets/asrm/asrm-content/news-and-publications/practice-guidelines/for-non-members/optimizing_natural_fertility.pdf
Vickram, A. S., Dhama, K., Chakraborty, S., et al. (2019). Role of Antisperm Antibodies in Infertility, Pregnancy, and Potential for Contraceptive and Antifertility Vaccine Designs: Research Progress and Pioneering Vision. Vaccines , 7 (3), 116. Retrieved from https://pubmed.ncbi.nlm.nih.gov/31527552/
Walker, M. H. & Tobler, K. J. (2021). Female Infertility. StatPearls. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK556033/
Wang, S., Zhang, Y., Mensah, V., et al. (2018). Discordant anti-müllerian hormone (AMH) and follicle-stimulating hormone (FSH) among women undergoing in vitro fertilization (IVF): which one is the better predictor for live birth? Journal of Ovarian Research , 11 (1), 60. Retrieved from https://link.springer.com/article/10.1186/s13048-018-0430-z#citeas
Zhang, J., Mu, X., Xia, Y., et al (2014). Metabolomic analysis reveals a unique urinary pattern in normozoospermic infertile men. Journal of Proteome Research , 13 (6), 3088–3099. Retrieved from https://pubs.acs.org/doi/abs/10.1021/pr5003142?source=cen